Call for papers Preregistration templates for toxicology and environmental health research

EBTC and the Center for Open Science are are inviting authors to submit “Preregistration Templates” to a Special Issue of Evidence-Based Toxicology.
Preregistration templates are a new type of manuscript designed to make it easier for researchers to reuse each others’ methods and gain the benefits of preregistering their studies.
Anyone with a method that more than one person might use should be able to create a preregistration template.
To submit, head to the EBT website, click “submit an article”, and from the options available in the submission portal select “Preregistration templates for toxicology and environmental health research”.
To find out more about what a preregistration template is and how to create one, read on! 👇
There will also be a webinar on 29 February for explaining the format and answering your questions. Sign up here!
What is a "preregistration template"?
A preregistration template is a form designed to help researchers specify the planned methods for their research before they collect their data. Similarly to study guidelines and reporting checklists, preregistration templates aim to improve how research is conducted and reported.
What makes preregistration templates different to guidance and checklists is they don’t just set out a standard for good practice around what a study should do and report: they provide step-by-step questions that help a researcher to complete all the tasks required for comprehensively planning their study. This step-by-step structure helps researchers be confident they have a near-complete plan for their required purpose before they commence data collection.
Anyone with a method that more than one person might use when planning a future study should be able to create a preregistration template. It is just a matter of getting the right level of detail and generalisability.
More information about the practices and value of preregistration, and why Evidence-Based Toxicology is supporting the development of preregistration templates, is available in the Special Issue editorial “Preregistration templates as a new addition to the evidence-based toxicology toolbox” (Mellor et al. 2024, coming soon!). Examples of preregistration templates can be found on the Open Science Framework, here. Guidance on what to include in a preregistration template submission is below.
---
How preregistration templates work
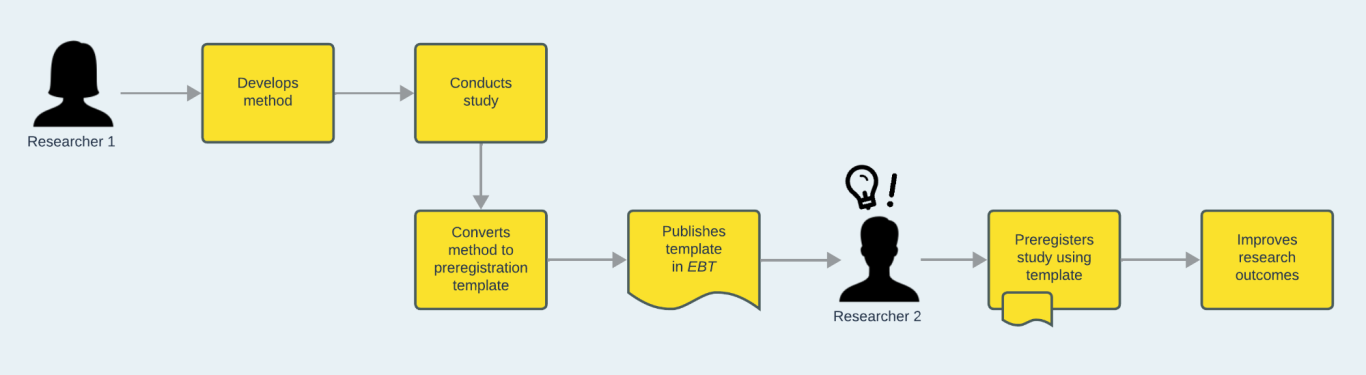
Researcher 1 develops a method for their study. Recognising that other researchers may benefit from a detailed operationalisation of their approach, they convert their method to a preregistration template. They have their template peer-reviewed and published, for example by a journal such as EBT. Researcher 2 discovers the template and uses it to preregister their own similar study, thus building on Researcher 1’s experience and gaining the benefits of preplanning their own work.
---
Overview of the Special Issue
This Special Issue is a joint effort between Evidence-Based Toxicology and the Center for Open Science, to support improvement in research by promoting the preregistration of scientific studies.
We will consider for the Special Issue any type of preregistration template in any suitable format, so long as it can be applied in the fields of toxicology or environmental health and it adheres to EBT’s submission guidelines around open data standards and the principles of evidence-based toxicology.
Submissions may include templates for new alternative methods (NAMs), animal studies, and human observational research, for both primary studies and secondary study designs such as systematic evidence maps and systematic reviews.
Authors should follow Evidence-Based Toxicology’s general instructions for authors, with the below instructions being specific to the Special Issue.
Note that the Special Issue will publish continuously and run through to midnight of 31 December 2024. Preregistration Templates will continue to be a submission type for Evidence-Based Toxicology after the Special Issue closes, the articles will just not be published as part of the Special Issue or be eligible for the prize for Best Preregistration Template.
Prize for Best Template
A prize for the best template submitted to the Special Issue will be awarded by the journal at the Society of Toxicology annual conference in 2025. The winner will be selected by the journal editors and the Center for Open Science, based on how well the template solves the problem it is designed to address, the rigour of the template development process, and the template’s application of open science and computable research principles.
Elements of a Preregistration Template submission
Preregistration Templates must first be submitted to the Evidence-Based Toxicology Community on Zenodo before being submitted via the journal submission portal.
The Zenodo submission must include the following:
- A cover letter using EBT’s template for preregistration templates (available here)
- The preregistration template manuscript (guidance on what to include is below)
- A declaration of interests (EBT’s preferred form available for download here)
For the journal submission portal, the Preregistration Template should be submitted to the Special Issue as a “Method” submission type.
Suggested Structure for a Preregistration Template
Preregistration templates are a new publication format and guidance for their optimal presentation and publication is likely to evolve rapidly. The structure below is therefore only a suggestion. Thoughtful deviations from the structure are encouraged.
Title: Describe as succinctly as possible the purpose and scope of the template, and include the phrase “a preregistration template” at the end of the title. For example:
- “Minimum information for registering the existence of a planned human epidemiological study: a preregistration template”
- “Planning the analysis of data generated in cell culture studies for assessing the toxicity of chemical exposures: a preregistration template”
- “A comprehensive study methods and data analysis plan for epidemiological studies that seek to comply with Good Epidemiology Practice: a preregistration template”
Context: Explain what gap the template fills, or what problem it solves. Describe how the template goes beyond currently available offerings, and how it is connected to any reporting checklists or study guidance that the template may be intending to support.
Template Purpose: Explain what is the purpose of the template. Give the reason for the chosen level of detail in relation to the purpose (templates may be concise or comprehensive, but there should be a rationale for one or the other). Describe the intended user and what tasks the template is intended to help the user complete. Provide evidence of current use or a justified estimate of potential use.
As examples, the purpose of a preregistration template would typically be one or more of the following:
- To increase transparency about the research process
- To constrain researcher degrees of freedom
- To specify a priori hypotheses
- To inform the community about the existence of a study
- To reduce duplicative research efforts
- Other (you tell us!)
Development Process: Describe how the template has been developed, including any user testing and community review or contribution process (e.g. expert input, community consultation, Delphi, focus groups) and/or literature-based methods that have informed the creation of the template. Also describe future development plans, if any.
User Guidance and Computability: Include a glossary of core terminology, guidance on how to use or interpret the template, and other information that may increase the likelihood of successful use of the template. Ontology integration, consideration around data exports, documentation support, and other application of computability principles in research design and reporting are encouraged.
The Template Itself: The template itself should of course be included in the manuscript, after the information above that provides the necessary contextual information for its evaluation and use.
The questions asked in a preregistration template will vary depending on its purpose but typically address one or more of the following planning issues:
- study design
- data collection procedures
- data analysis plans
- the criteria by which inferences and conclusions will be drawn from the data
Ideas for Getting Started
Preregistration templates that can support researchers in following best practices in planning, conducting, and reporting research would be valuable in many areas of toxicology and environmental health research. We could suggest the following topics, for those looking to get started:
New and Alternative Methods (NAMs). This is a fast-growing space with proliferating study guidance and reporting checklists, that can be challenging to interpret into study protocols or plans.
Epidemiology. Environmental health epidemiology studies are seldom preregistered. High-quality preregistration templates may help researchers plan to collect data that may increase their acceptance in regulatory risk assessment but might otherwise be overlooked.
Systematic reviews and systematic evidence maps. Preregistration of SRs and SEMs is still uncommon and frequently overlooks important steps of the synthesis process. Preregistration templates may help researchers plan rigorous methods for one or more stages of the SR or SEM process, particularly if they are specifically tailored to toxicology or environmental health contexts.
Review Process for Preregistration Templates
Preregistration Templates submitted for the Special Issue will be evaluated according to the following process:
1. An initial triage review focused on compliance with EBT’s open science standards will be conducted by the editors of EBT, with comments posted on Zenodo and shared with authors as per EBT’s open review policies.
2. On clearing triage, the preregistration template will be reviewed by a team of EBT peer-reviewers and reviewers from the Center for Open Science Preregistration Template Working Group;
3. The reviewer comments will be compiled into an evaluation report that will be posted on Zenodo and shared with the authors, as per EBT’s open review policies;
4. Once reviewers, authors, and handling editor are satisfied that the template is a sufficient contribution to the scientific literature, the template will be accepted by EBT (following the standard consensus-based decision-making process for the journal);
5. Separately, the Center for Open Science Preregistration Template Working Group will make a recommendation as to whether the template be approved as a “supported template” on the Open Science Framework (this may involve additional comments in relation to technical requirements for template support - see here for more information).
Evidence-Based Toxicology Collaboration at
Johns Hopkins Bloomberg School of Public Health
615 N. Wolfe Street, Baltimore, MD 21205
Website content issued under a CC-BY license
We need your consent to load the translations
We use a third-party service to translate the website content that may collect data about your activity. Please review the details in the privacy policy and accept the service to view the translations.
